Electron Configuration of Silicon in Excited State
Electron configuration of Silicon is Ne 3s2 3p2. Base your answers to questions 52 and 53 on the information below.
![]()
Silicon Orbital Diagram Electron Configuration And Valence Electrons
The electron configuration of silicon in excited state is Si14 1s 2 2s 2 2p 6 3s 1 3p x 1 3p y 1 3p z 1.
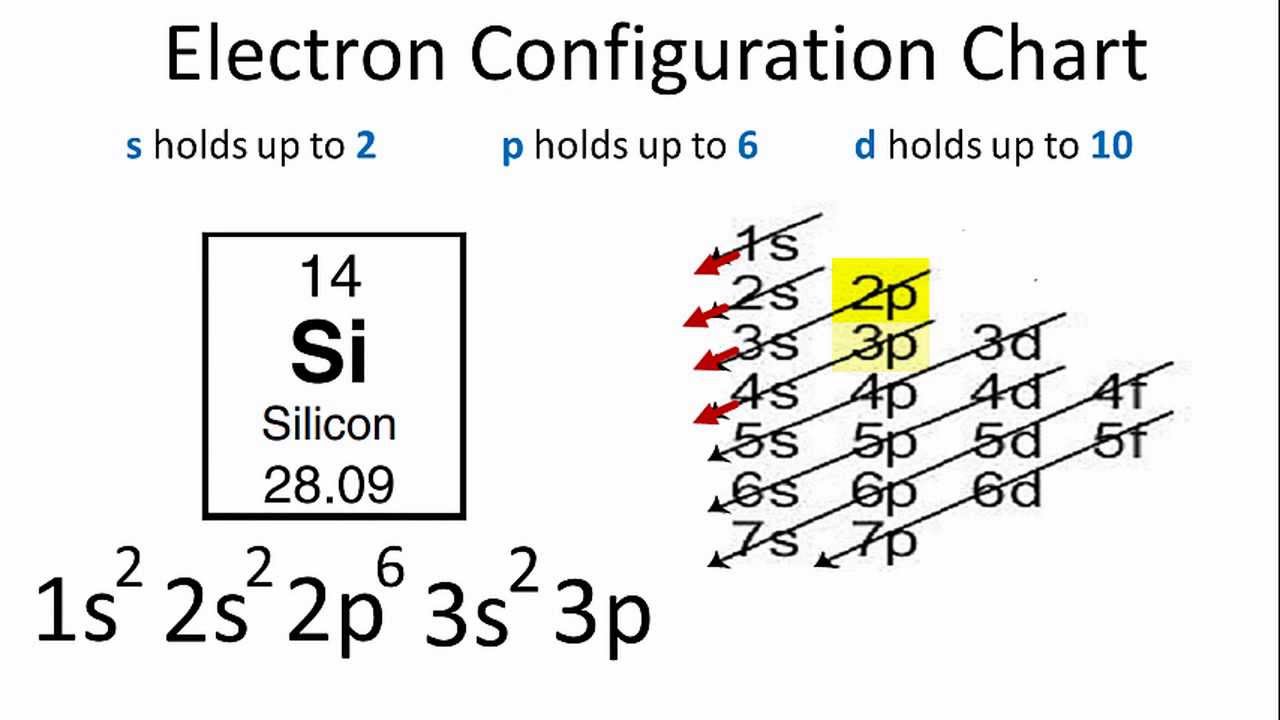
. The electronic configuration of silicon is. Since the 3s if now full well move to the 3p where well place the remaining two electrons. On heat do 2 pts Based on the Hunds rules what is the lowest energy.
Silicon has an electron configuration of 1s2 2s2 2p6 3s2 3p2. The first excited electronic configuration is to promote one 4s electron into the 3d orbitals. 1 Highlight box for Answer.
2 days agoHence in excited state one of the 2s electron will jump to 2p orbitalso the excited state electronic configuration should be 1s2 2s1 2px2 2py1 2pz1. Silicon is a chemical element with atomic number 14 which means there are 14 protons and 14 electrons in the atomic structure. There are still 14 electrons making it a neutral silicon atom.
Question 19 N 1 pts Which of the following could be an electron configuration for an excited state silicon Si. And the second excited state configuration regardless of the different spectroscopic terms and sublevels is to promote the 4s electron into 4p. Here this electron configuration shows that the last shell of the silicon atom has four unpaired electrons3s 1 3p x 1 3p y 1 3p z 1.
Jul 26 2014. Using the noble gas notation the electron configuration of silicon can be denoted by Ne 3s 2 3p 2. The ground state electron configuration for Silicon is.
The p orbital can hold up to six electrons. The nex six electrons will go in the 2p orbital. For example an atom in an excited state may contain two electrons in its 1s orbital one electron in its 2s orbital and one electron.
I Since the electronic configuration of Si is given as Si - Ne 3s2 3p2 If electron is excited from 3p orbital it will goes in 4s orb View the full answer Transcribed image text. The valency of the element is determined by electron configuration in the excited state. Electronic transitions involving rearrangement of valence electrons.
So any electron configuration in which the last electron again the valence electron is in a higher energy orbital this element is said to be in an excited state. In this case the valency of silicon is 4. Need help with chemistry.
The abbreviated form is Ne 3s2 3p2. Energy level diagrams and the hydrogen atom. SiZ14 1s 2 2s 2 2p 6 3s 2 3p 2.
An excited state electron configuration refers to an atom with electrons at a higher energy level than is necessary. B 1 pt Identify all possible values of L for it. It contains 14 protons and 14 electrons with.
There are 14 electrons in a silicon atom. An excited state means that typically the valence electron has moved from its ground state orbital ie. Silicon atoms have 14 electrons and the shell structure is 284.
Based on rules for filling of electrons in various orbitals. School of Chemistry and Biochemistry Georgia Institute of Technology. The ground state electron configuration of ground state gaseous neutral silicon is Ne.
What is the electron configuration for silicon. The electron configuration of an atom is important as it helps to predict the chemical electrical and magnetic behavior of substance. In the periodic table of elements silicon is represented by the chemical symbol Si atomic number 14 and relative atomic mass of 28085.
Well put six in the 2p orbital and then put the next two electrons in the 3s. 3p2 and the term symbol is 3P0. Silicon has the electron configuration of 1s2 2s2 2p6 3s2 3p2.
Electronic configuration of Si in excited state is 1s 2 2s 2 2p 6 3S 2 3p x 1 3 p y 1 3 p z 1 Silicon undergoes sp 3 hybridization and forms four half-filled sp3 hybrid orbitals which are used to form four covalent bonds. 52 Identify one element from this table for each type of element. Although the element can be found all over the world it is not found in our environment individually.
The excited state configuration I would write is 1s22s22p53s23p25s1. The excited-state electron configuration for Silicon is 1s 2 2s 2 2p 6 3s 1 3p 3 or 1s 2 2s 2 2p 6 3s 2 3p 1 4s 1. Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2.
79 pts Consider an excited state electron configuration of silicon Ne38 3p5g a 1 pt Identify all possible values of S for it. Electron Configuration and Oxidation States of Silicon. A neutral atom of Ne 3s23p2 O Ne 3523p451 O Ne 3s23p2451 O Ne 3s23p3.
3 pts Identify all possible terms that can arise from this electron configuration. Then the electron configuration of siliconSi in excited state will be 1s 2 2s 2 2p 6 3s 1 3p x 1 3p y 1 3p z 1. 51 In your answer booklet write an electron configuration for a silicon atom in an excited state.
1s 2 2s 2 2p 6 3s 2 3p 2. Lowest available energy to some other higher energy orbital. Metal metalloid and nonmetal.
This electron configuration shows that the last shell of the silicon atom has four unpaired electrons. Possible oxidation states are -4. One example of an electron configuration for an excited electron of silicon would be eqmathrm1s22s22p53s23p3 eq.
The excited-state configuration of an atom is different from the regular configuration of an atom this occurs when an electron is excited and jumps into a higher orbital. An excited state differs from a ground state which is when all of the atoms electrons are in the their lowest possible orbital. The chemical symbol for Silicon is Si.
So while in general predicting electronic configurations for excited states is a tricky business you are right. Ultraviolet visible UVvis spectra are dominated by electronic transitions Electronic transitions typically occur in the 1-12 eV range 10000-100000 cm-1 10000-50000 cm-1.

Silicon Si Electron Configuration And Orbital Diagram

Silicon Electron Configuration Si With Orbital Diagram
![]()
Silicon Orbital Diagram Electron Configuration And Valence Electrons
![]()
Silicon Occurrence Preparation Physical And Chemical Properties Uses
No comments for "Electron Configuration of Silicon in Excited State"
Post a Comment